Research Projects
Novel regulators of β-Cell proliferation and function
Regulation of β-Cell proliferation and function by Tead1 and the Hippo pathway
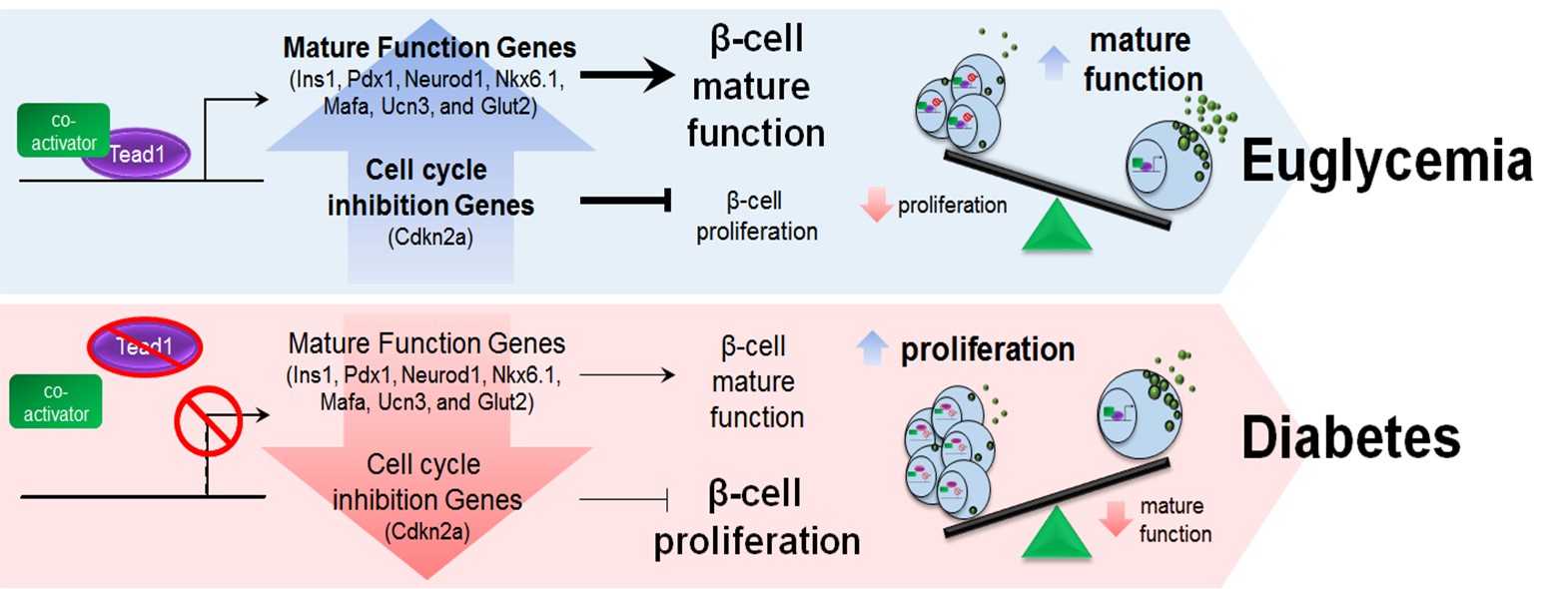
Our group has identified Tead1, the Hippo effector, as a non-redundant reciprocal regulator of the proliferative drive and functional competence in β-cells. Differential modulation of these activities may allow an increase in functional β-cell mass without a loss of critical glucose homeostatic function as a therapeutic approach to diabetes.
Example Publication(s):
Lee, J et al. Tead1 reciprocally regulates adult β-cell proliferation and function to maintain glucose homeostasis
doi: https://doi.org/10.7554/eLife.95603.1
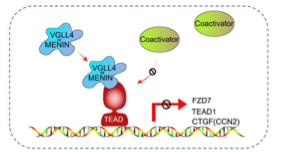
We have discovered that VGLL4 and MENIN bind to the C-terminal pocket region of TEAD1 and competitively inhibit co-activation of TEAD1, leading to a repression of β cell proliferation-related genes. The balance between coactivators and these newly identified corepressors may play essential roles in modulating TEAD pathway activity and β cell proliferation and maturity.
Example Publication(s):
Li, F et al. VGLL4, and MENIN function as TEAD1 corepressors to block pancreatic β-cell proliferation. Cell Rep. 2023 Jan 31;42(1):111904. doi: 10.1016/j.celrep.2022.111904
TEAD1 also represses transcription in a pocket-independent manner, in part by preventing the binding of POLII to the TSS on the promoter. We identified a transcriptional repressive mechanism of TEAD1 that is widely prevalent in cells that are normal, highly differentiated cells, and those that are malignant.
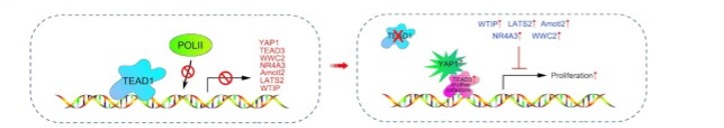
Li, F et al. TEAD1 regulates cell proliferation through a pocket-independent transcription repression mechanism. Nucleic Acids Res. 2022 Dec 9;50(22):12723-12738. doi: 10.1093/nar/gkac1063
Epigenetic regulation of β-Cell proliferation and function
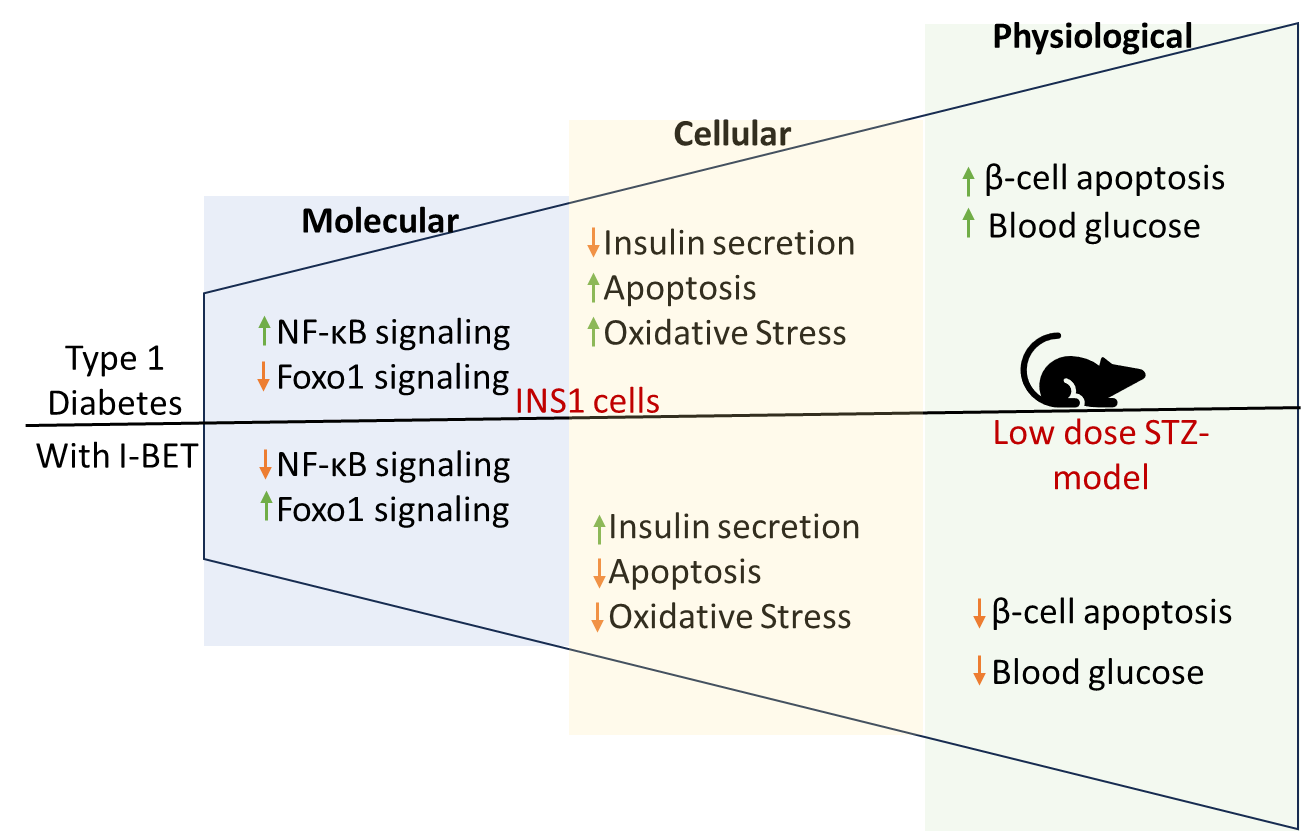
The bromodomain-containing BET (Bromodomain and extra-terminal domain containing) proteins, including BRD2, BRD3, BRD4, and BRDT, are epigenetic regulators of gene transcription known to be involved in inflammatory response, proliferation, apoptosis, and differentiation. These proteins bind to the acetylated lysine of histones in the genome, acting as a ‘reader’ and recruit histone remodeling proteins, such as HATs (histone acetyltransferase), HDACs (histone deacetylase), and transcription elongation factors. Recently, with the advent of a new generation of higher affinity chemical inhibitors such as I-BET, there has been a surge in testing their therapeutic utility for many oncological (prostate, breast, hematopoietic glioblastoma) and vascular (pulmonary arterial hypertension) diseases despite the limited knowledge of underlying molecular mechanism; I-BET primarily targets BRD2, 3, and 4. Thus, to understand its potential therapeutic potential in T1D, we determined the effect of BET inhibition on protecting β-cell function and identify underlying molecular mechanisms. I-BET treatment not only enhanced critical factors that promoted β-cell function but also antagonized the cytokine-induced upregulation of the NF-kB pathway that is known to induce apoptosis in pancreatic β-cells in T1D.
Example Publication(s):
Negi V et al. Bromodomain protein inhibition protects β-cells from cytokine-induced death and dysfunction via antagonism of NF-κB pathway. Cells 2024 June 26;13 (13):1308. doi: 10.3390/cells13131108
Circadian clock regulation of metabolism and stress pathways and their role in diabetes, obesity, heart failure, and atherovascular disease
Regulation of β-Cell function by the Circadian Clock
The circadian clock has been shown to regulate metabolic homeostasis. Mice with a deletion of Bmal1, a key component of the core molecular clock, develop hyperglycemia and hypoinsulinemia, suggesting β-cell dysfunction. Loss of Bmal1 in β-cells leads to diabetes secondary to a significant impairment in glucose-stimulated insulin secretion (GSIS), but not to other depolarizing secretagogues. This defect in GSIS occurs as a result of changes in antioxidant gene expression which are under direct Bmal1 transcriptional control, and oxidative stress-induced uncoupling due to an upregulation of Ucp2 and mitochondrial dysfunction. Bmal1 regulates mitochondrial energy metabolism to maintain normal GSIS, and its disruption leads to diabetes due to a loss of GSIS.
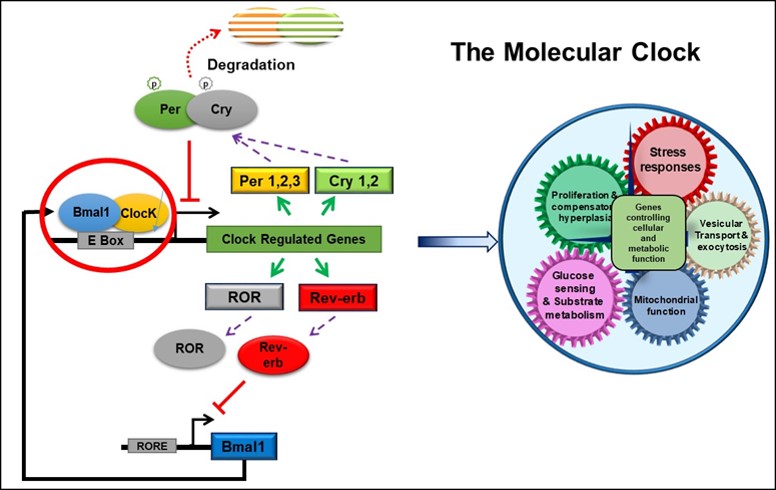
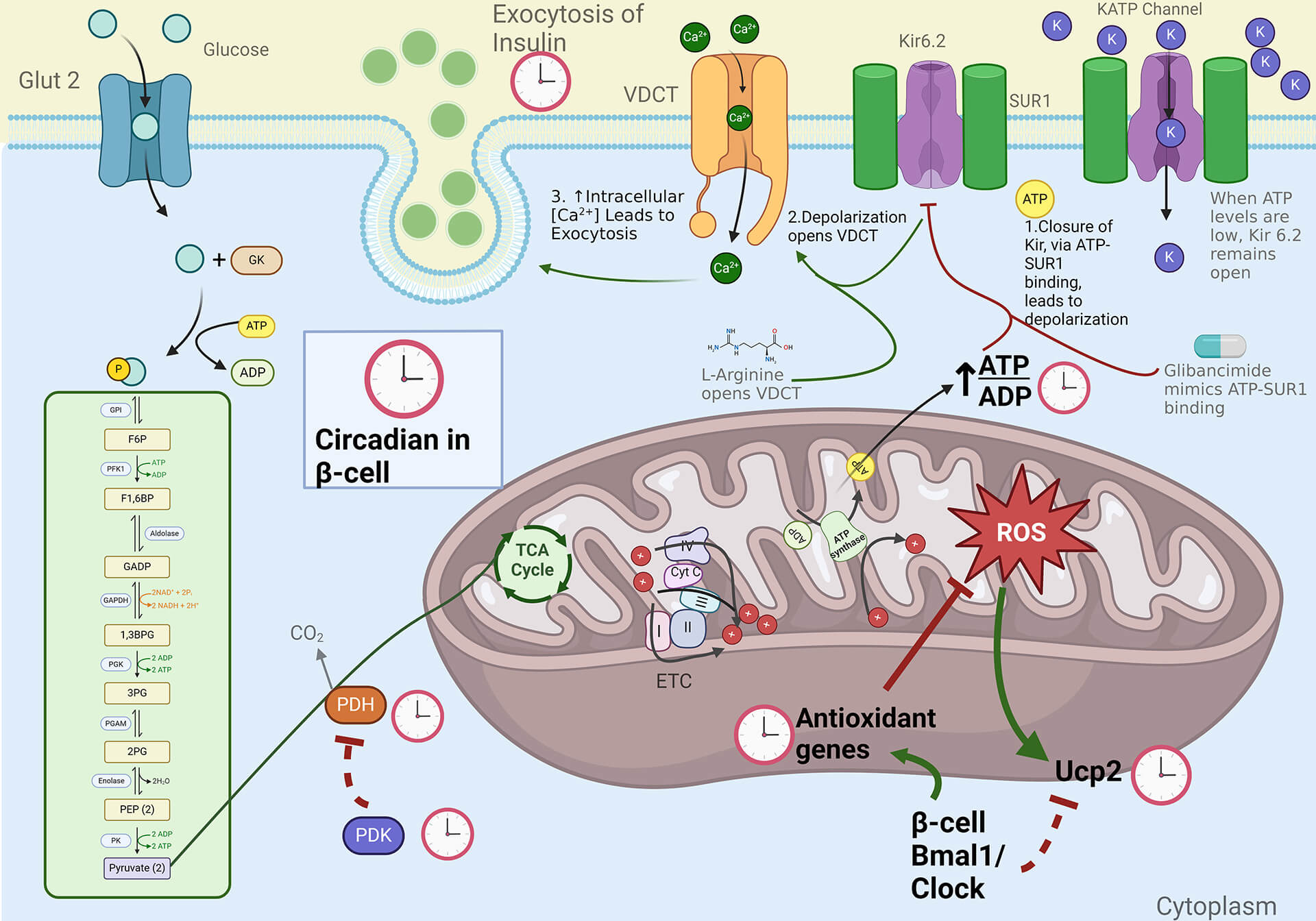
Role of Circadian Clock Disruption in the Pathogenesis of Heart Failure
Regulation of Atherovascular Disease and Metabolic Syndrome by the Circadian Clock
As part of a multi-institutional collaborative effort between the University of Pittsburgh, Mt. Sinai School of Medicine, and Albany Medical College, we are examining the effects of disruptive environmental light patterns in atherosclerosis and diabetes risk. We are evaluating the use of unique light patterns and circadian molecular clock pharmacological agonists to reduce excess disease risk in shift workers.
Example Publication(s):
Figueiro M, Goo Y, Hogan R, Plitnick B, Lee J, Jahangir K, Moulik M, Yechoor V*, Paul A. Light-Dark Patterns Mirroring Shift Work Accelerate Atherosclerosis and Promote Vulnerable Lesion Phenotypes. J Am Heart Assoc 2021 Jan 19;10(2):e018151. doi: 10.1161/JAHA.120.018151. Epub 2021 Jan 6. *Corresponding and co-senior author.
